FWF / Project P 20461: “Biodiversity of Marine Tintinnids (Protista, Ciliophora)” – Grant amount: € 248.590. Period: 01.05.2008–01.05.2011
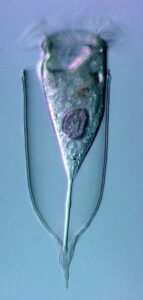
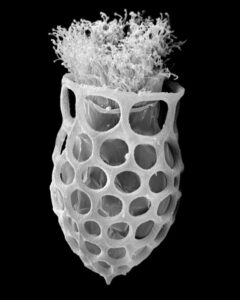
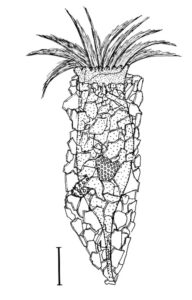
New morphologic and genetic features have been discovered in tintinnid ciliates, microscopic unicellular animals with minute, hair-like appendages, collected in the Mediterranean and North Atlantic. The data contribute to a better understanding of the species limits and the evolution of tintinnids and their relationships. Tintinnids seem to be an important component of the marine planktonic food web as they feed on bacteria and toxic algae and are preyed upon by small multicellular animals (e.g., fish larvae). The main feature of tintinnids is the 20–1000 µm long lorica (house), which is vase-shaped or tubular and has a hyaline (clear) or agglomerated (covered with environmental particles) structure. Since their discovery in the year 1776, about 1200 species in 75 genera and 15 families have been described, using merely lorica features, which turned out to be unsuitable for a natural classification, as species often form different kinds of houses. Reliable features are, however, poorly known, viz., cell morphology and genes have been studied in only 3 % of the species.
During the project, a membranous sac, that closes in disturbed specimens, was found in the loricae of four genera belonging to three families. Since live observation and scanning electron microscopy revealed an identical ultrastructure and functionality of the sac, indicating a common origin, the four genera were united in one family despite distinct differences in their lorica structures.
In gene analyses, the genus Favella formed two distantly related groups. Now, light and electron microscopy showed conspicuous differences in their cell features and lorica wall ultrastructures. Hence, the genus Favella was split, a new genus was established, and the 39 species were assigned.
In nature, foreign particles hide the lorica matrix of Tintinnopsis parvula. Houses formed in particle-free cultures, however, revealed the wall matrix, which differed from that found in related species. The wall ultrastructure thus represents a promising feature for a split of the species-rich genus Tintinnopsis in the future. Using modern methods, this and further insufficiently known species were redescribed based on several populations each. Additionally, 16 new gene sequences were obtained to study the temporal and spatial variability within and the relationships between tintinnid species. The sedimentation of loricae represents a huge material flux in deep water layers. To understand the remineralization and fossilization processes, the chemical composition of the houses was studied by diverse staining methods, enzyme experiments, and extreme transmission electron microscopy (resolution < 20 nanometre). The interpretation of morphologic features in tintinnids often requires a comparison with their naked relatives. In both groups, the evolution and relationships can perfectly be inferred from certain cell features and genes. Although the majority of these organisms probably has a cosmopolitan distribution in the sea, some species are very likely endemic and thus should not any longer be excluded from conservation issues.
Link zu FWF-Projekt P 20461
Project associated publications:
- Agatha S. (2010): A light and scanning electron microscopic study of the closing apparatus in tintinnid ciliates (Ciliophora, Spirotricha, Tintinnina): a forgotten synapomorphy. J. Eukaryot. Microbiol. 57: 297–307. https://doi.org/10.1111/j.1550-7408.2010.00490.x
- Agatha S. (2010): Redescription of Tintinnopsis parvula Jörgensen, 1912 (Ciliophora: Spirotrichea: Tintinnina), including a novel lorica matrix. Acta Protozool. 49: 213–234.
- Agatha S. (2011): Updated hypothesis about the evolution of oligotrichid ciliates (Ciliophora, Spirotricha, Oligotrichida) based on somatic ciliary patterns and ontogenetic data. Eur. J. Protistol. 47: 51–56. https://doi.org/10.1016/j.ejop.2010.09.001
- Agatha S. (2011): Global diversity of aloricate Oligotrichea (Protista, Ciliophora, Spirotrichea) in marine and brackish sea water. PLoS ONE 6: e22466 https://doi.org/10.1371/journal.pone.0022466
- Agatha S. & Foissner W. (2009): Conjugation in the spirotrich ciliate Halteria grandinella (Müller, 1773) Dujardin, 1841 (Protozoa, Ciliophora) and its phylogenetic implications. Eur. J. Protistol. 45: 51–63. https://doi.org/10.1016/j.ejop.2008.07.004
- Agatha S. & Strüder-Kypke M.C. (2012): Reconciling cladistic and genetic analyses in choreotrichid ciliates (Ciliophora, Spirotricha, Oligotrichea). J. Eukaryot. Microbiol. 59: 325-350. https://doi.org/10.1111/j.1550-7408.2012.00623.x
- Agatha S. & Tsai S.-F. (2008): Redescription of the tintinnid Stenosemella pacifica Kofoid and Campbell, 1929 (Ciliophora, Spirotricha) based on live observation, protargol impregnation, and scanning electron microscopy. J. Eukaryot. Microbiol. 55: 75–85.doi: 10.1111/j.1550-7408.2008.00309.x
- Foissner W., Stoeck T., Agatha S. & Dunthorn M. (2011): Intraclass evolution and classification of the Colpodea (Ciliophora). J. Eukaryot. Microbiol. 58: 397–415. https://doi.org/10.1111/j.1550-7408.2011.00566.x
© Sabine Agatha All Rights Reserved




