Intergranular Regions Inside Nanocrystalline Ceramics
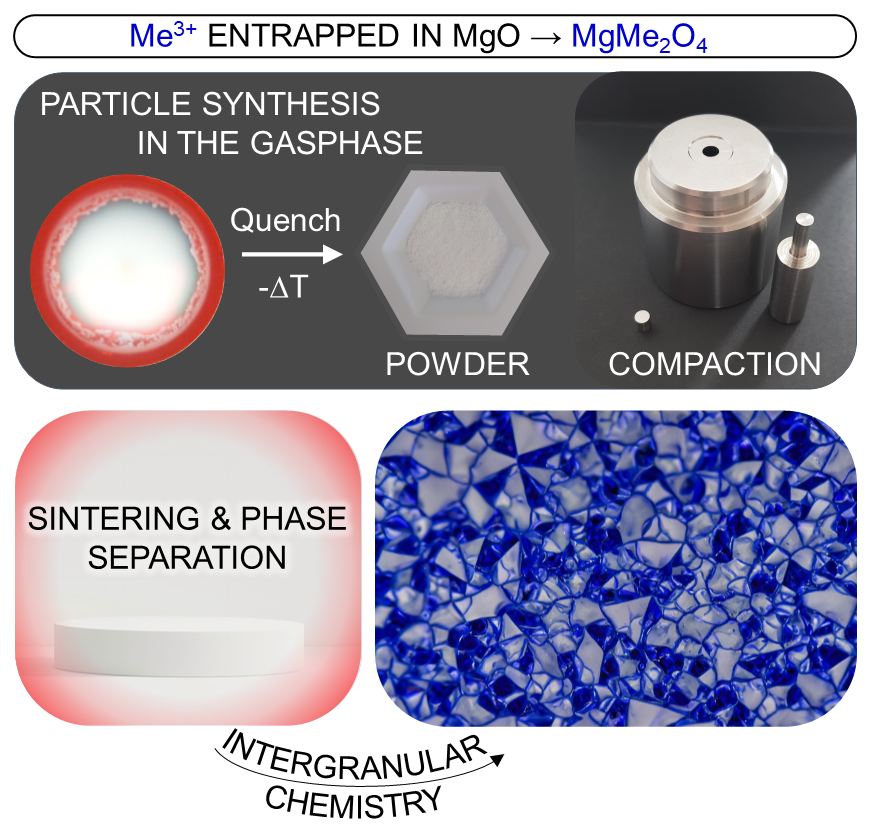
Functional ceramics represent an irreplaceable class of materials spanning a wide application range from microelectronics, inorganic phosphors up to material components for energy conversion. Structural variety and possible material combinations made nanostructured metal oxide composites to promising candidates for the design of ceramic materials. Their macroscopic properties are controlled by the constituent grains and equally important by the intergranular region in between them. The advancement of functionality of related materials critically requires the mechanistic understanding of underlying transformations during processing over a variety of length scales.
Schematic description of our approach towards functional material properties with designed intergranular regions. Starting with the introduction of impurities during gas phase synthesis of particles, involves dedicated processing protocols to control segregation and interface chemistry at different length scales and thus the dispersion of admixtures inside the ceramic microstructure. © Thomas Schwab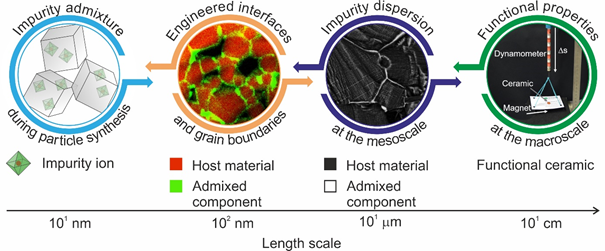
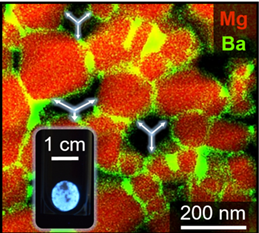
Segregation engineering using Ca2+– and Ba2+-ions inside MgO host nanoparticles controls surface and interface energetics and, thus, grain coarsening and microstructure evolution during sintering, whereby the dispersion of dopants over the intergranular region provides means to retain and adjust alkaline earth oxide (AEO) specific photoluminescence emission features from pores and outer surfaces.
Current and former collaborators on the project:
Hasan Razouq, MSc.
Ellie Neige, MSc.
Korbinian Aicher, MSc.
Antonios Litovoilis, BSc.
Daniel Thomele, MSc.
Matthias Niedermaier, PhD.
Thomas Schwab, PhD.
Publications
Schwab Thomas, Aicher Korbinian, Zickler Gregor A., Reissner, M., and Oliver Diwald, Inside Ceramics and Between MgO Grains: Solid-State Synthesis of Intergranular Semiconducting or Magnetic Spinels; Small Methods, 2024, DOI: 10.1002/smtd.202400715
Schwab, Thomas, Thomele, Korbinian Aicher, John W. C. Dunlop, Keith McKenna, and Oliver Diwald, Rubbing Powders: Direct Spectroscopic Observation of Triboinduced Oxygen Radical Formation in MgO Nanocube Ensembles, J. Phys. Chem. C, 2021, 125, 40, 22239-22248.DOI: 10.1021/acs.jpcc.1c05898
Thomele, D, Baumann, S.O., Schneider, J., Sternig, S.K., Shulda, S., Richards, R.M., Schwab, T., Zickler, G.A., Zickler, Bourret, G.R, and Diwald, O., Cubes to Cubes: Organization of MgO Particles into One-Dimensional and Two-Dimensional Nanostructures, Cryst. Growth Des. 2021, 21, 8, 4674-4682. DOI:10.1021/acs.cgd.1c00535
Schwab, K. Aicher, H. Razouq, G. Zickler, O. Diwald, Segregation engineering in MgO nanoparticle derived ceramics: The impact of calcium and barium admixture on coarsening and light emission properties, Appl. Mater. Interf. 2021, 13, 21, 25493-25502. DOI: 10.1021/acsami.1c02931
Schwab, M. Niedermaier, K. Aicher, M. Elsässer, G. Zickler, O. Diwald, Always cubes: A comparative evaluation of gas phase synthesis methods and precursor selection for the production of MgO nanoparticles, Open Ceram.2021, 6, 100104. DOI: 10.1016/j.oceram.2021.100104
Schwab, H. Razouq, K. Aicher, G. Zickler, O. Diwald, Apparent crystallite domain size growth in metal oxide nanocrystal ensembles: The importance of surface reactivity of powders for processing, Open Ceram. 2020, DOI: 10.1016/j.oceram.2020.100014 .
Schwab, M. Niedermaier, G. Zickler, M. Ončák, O. Diwald, Isolated cobalt ions embedded in magnesium oxide nanostructures: Spectroscopic properties and redox activity, Chem. Eur. J. 2020, DOI: 10.1002/chem.202002817.
Niedermaier, T. Schwab, P. Kube, G. A. Zickler, A. Trunschke, O. Diwald, Catalytic activity, water formation, and sintering: Methane activation over Co- and Fe-doped MgO nanocrystals, J. Chem. Phys. 2020, 152, 074713.
Bourret, O. Diwald, Thin water films covering oxide nanomaterials: Stability issues and influences on material processing, J. Mater. Res. 2019, 34, 428-441.
Niedermaier, T. Schwab, P. Dolcet, J. Bernardi, S. Gross, M. Bockstedte, O. Diwald, Cobalt and iron ions in MgO nanocrystals: Should they stay or should they go, J. Phys. Chem. C 2019, 123, 25991–26004.
Niedermaier, C. Taniteerawong, T. Schwab, G. Zickler, J. Bernardi, O. Diwald, Impurity segregation and nanoparticle reorganization of indium doped MgO cubes, ChemNanoMat 2019, 5, 634-641.
Thomele, A. Gheisi, M. Niedermaier, M. Elsässer, J. Bernardi, H. Grönbeck, O. Diwald, Thin water films and particle morphology evolution in nanocrystalline MgO, J. Am. Ceram. Soc. 2018, 101, 4994-5003.
Thomele, G. Bourret, J. Bernardi, M. Bockstedte, O. Diwald, Hydroxylation induced alignment of metal oxide nanocubes, Angew. Chem. Int. Ed. 2017, 56, 1407-1410.
Niedermaier, P. Dolcet, A. R. Gheisi, G. Tippelt, W. Lottermoser, S. Gross, J. Bernardi, O. Diwald, Stability and local environment of iron in vapor phase grown MgO nanocrystals, J. Phys. Chem. C 2017, 121, 24292–24301




