Molecular and Cellular Allergology

Allergies affect up to a third of the world’s population. About 15-20% of patients experience severe and debilitating disease. Further, most allergic conditions start already in childhood and peak during the highly productive years of an individual. For instance, allergic rhinitis affects up to 45% of Europeans aged 20–40 years. Allergies to pollen are the most frequent type of allergy, with an estimated prevalence of 40% among 100 million Europeans suffering from respiratory allergies. In addition, exposure to pollen was shown to be a risk factor for infections by respiratory viruses, in both allergic and non-allergic individuals.
The underlying immune response has two distinct phases: (i) sensitization phase, i.e. the sub-clinical/asymptomatic immune response to exposure to an allergen source, and (ii) effector phase, i.e. disease expression or pathophysiology. Genetic predisposition, the type of allergen/allergen source, the level of allergen exposure, and a concomitant exposure with adjuvants (microbial contaminants, environment pollutants) affect sensitization. Understanding the molecular mechanism involved in the interaction of allergen source with the relevant structural and immune cells is crucial for therapeutic interventions. Over the years, our group has focused on the molecular and immunological characterization of birch, ragweed, and mugwort pollen allergens. Currently, we pursue several lines of research aiming to unravel the intrinsic and extrinsic factors in allergen sources involved in the initiation of sensitization and Th2-biased immune responses to allergens. We are also interested in developing novel approaches for tolerance induction in allergen-specific immunotherapy.
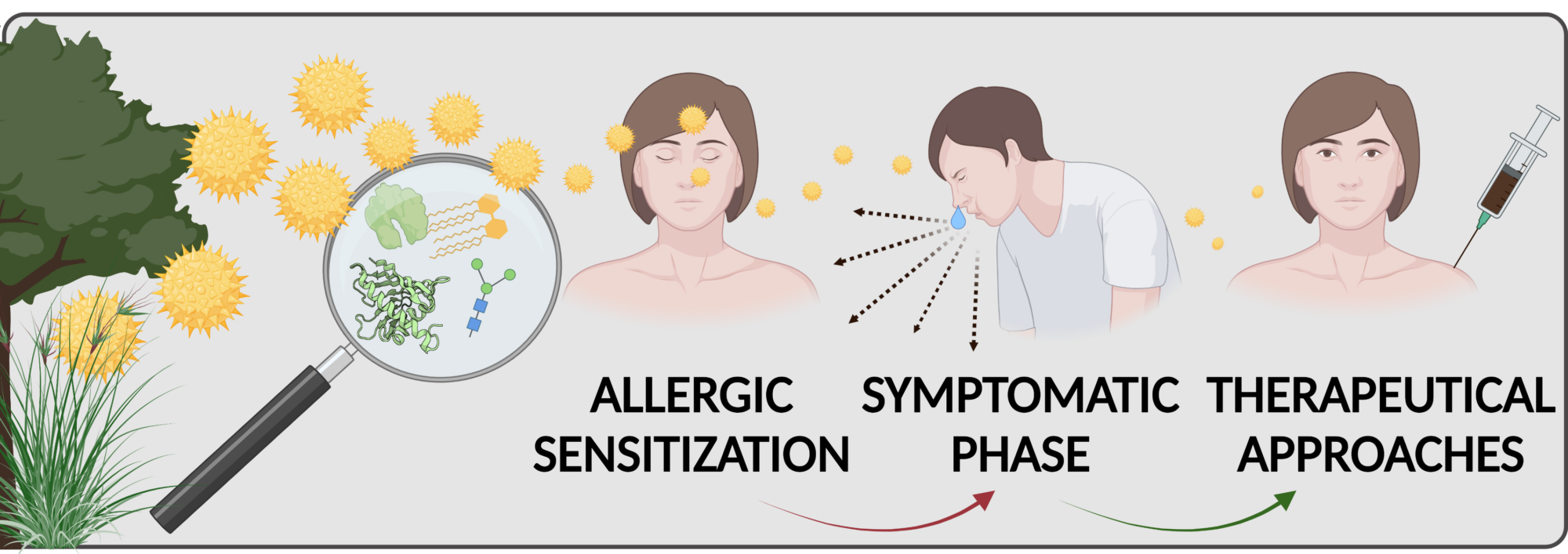
Mechanisms of initiation of sensitization by allergenic pollen
We have demonstrated that aqueous extracts of birch pollen, even in the absence of added adjuvants, have the capacity to induce Th2 allergic inflammation. In contrast, purified Bet v 1 (the major birch pollen allergen) alone is a very weak immunogen regarding the activation of dendritic cells and the polarization of T helper cells. These findings indicate that Bet v 1 lacks inherent sensitizing potential and that the pollen context (pollen matrix) empowers the molecule with its full allergenic potential. Thus, we hypothesized that sensitization to Bet v 1 results from a pre-primed Th2 micromilieu initiated by pollen-derived adjuvant components. To identify the immunomodulatory/adjuvant compounds we use biochemical fractionation and cell-based screening assays. We are particularly focusing on innate immune receptors such as Toll-like receptors and their signaling pathways.
Novel approaches for tolerance induction in allergen-specific immunotherapy (AIT)
Within an EU-funded first-in-men clinical trial ( www.bm4sit.eu), we have recently investigated the efficacy of a novel birch pollen vaccine based on BM4, a hypoallergenic Bet v 1 derivative that has been developed and patented by our research group. We also participated in a clinical trial investigating whether the co-administration of VitD3 is having a beneficial effect on a commonly used birch pollen AIT vaccine. In course of the two mentioned clinical trials, we are also analyzing epigenetic alterations induced by AIT using a genome-wide DNA methylation approach (collaboration with Angelika Lanhsteiner and Angela Risch in our Department). Analyzing these epigenetic modifications might provide new insights into the underlying mechanisms induced by AIT as well as by the used alum adjuvant. For an alternative treatment approach, we are testing Bet v 1 and Phl p 5 in combination with TGF-β and/or IL-10 mimetic peptides/proteins to provide an anti-inflammatory micromilieu that may shift the immune response towards Tregs and tolerance against the allergen. As far as adjuvant research is concerned, we are investigating the usage of silica particles (at the micro and nanoscale) as novel adjuvant candidates for AIT (collaboration with Albert Duschl and Martin Himly in our Department). We are studying the immune responses induced by preparations of soluble allergen compared to allergen coupled to silica particles in allergic sensitization models allergenicity as well as in therapeutic in vivo models in order to understand how solubility affects allergenicity and immunogenicity.
NEWS * NEWS * NEWS*
We are super excited to congratulate Lisa Pointner for winning the 1st place of the Young Investigators Award 2021 of the Paris Lodron University of Salzburg! Congratulations!!!

We are happy to announce here our most recent publications:
- Birch Pollen Induces Toll-Like Receptor 4-Dependent Dendritic Cell Activation Favoring T Cell Responses
- Humanized Mediator Release Assay as a Read-Out for Allergen Potency
- Structural Alterations of Antigens at the Material Interface: An Early Decision Toolbox Facilitating Safe-by-Design Nanovaccine Development
Information on COVID-19 vaccines provided by the OpenScience platform:
How do they work?
https://www.openscience.or.at/de/wissen/medizin-mensch-ernaehrung/2022-03-03-videos-zu-sars-cov-2/
EAACI explains vaccinations
To vaccinate or not to vaccinate? This question raises a great deal of discussion. To find out what a group of international scientists and experts of the immune system have to say about it, take a look at our short and informative animated video.
Links to Allergy and Immunology Societies:
- European Academy of Allergy and Clinical Immunology (EAACI)
- The Austrian Society for Allergology and Immunology (ÖGAI)
- Austrian Association of Molecular Life Sciences and Biotechnology (ÖGMBT)
- European Federation of Immunological Societies (EFIS)
The research group is a member of the FWF-funded international PhD Program “Immunity in Cancer and Allergy” (ICA), member of the doctoral school PLUS “Biomolecules” and a member of the “ Allergy-Cancer-BioNano Research Centre” (ACBN) from the Paris Lodron University of Salzburg.




